In Silico and AI: Computer Simulation in Drug Discovery
In a sign of gathering confidence and support from healthcare players and the industry overall, total funding from 2019 to 2020 for in silico clinical trials and drug discovery reached $750 million. The sum for the technology—which utilizes computer modeling and simulation on virtual patients for drug testing, instead of employing live subjects or specimens—represents funding for seven pharmaceutical companies, the largest chunk of which involves $280 million for Canadian outfit AbCellera Biologics.
In early October, biosimulation software solutions maker Certara announced it had submitted a Form S-1 registration statement with the US Securities and Exchange Commission in connection with the proposed initial public offering of the company’s common stock. The move by New Jersey-based Certara follows similar but separate efforts from its other rivals to secure more funding for in silico drug discovery and clinical trials.
Recent interest toward in silico dates to July 2019, when leading precision medicine company GNS Healthcare, headquartered in Massachusetts, announced a $23 million Series D fundraising round, led by Cigna Ventures, a strategic corporate venture capital partner and wholly owned indirect subsidiary of Cigna Corporation.
Three months later, in October 2019, German biotechnology company BioNTech raised $150 million in its IPO, which came up short of what it had expected. However, the drug maker’s involvement in an experimental mRNA COVID-19 vaccine, developed with US firm Pfizer, caused its stock value to triple in value in June 2020.
In January this year, French health-tech company Novadiscovery closed the first 5 million euros—out of a total 7 million euros—Series A funding round with Swiss entity Debiopharm. A month later in February, in silico drug discovery company Schrodinger raised $232.3 million in proceeds from its IPO, opening at $17 per share.
In May 2020, UK-based Exscientia raised $60 million in a series C financing round led by Novo Holdings, the wholly owned holding company of Danish diabetes medicine maker Novo Nordisk, along with German drug development company Evotec, US pharmaceutical company Bristol Myers Squibb, and Asian-based private investment partnership GT Healthcare Capital. This brought the company’s total funding to just over $100 million. The new capital will be used in part to expand the company’s AI capabilities in biology.
That same month, AbCellera Biologics received a funding round of $105 million that included additional investment from Eli Lilly, the Indiana-based pharmaceutical giant that was the first to mass-produce insulin and the polio vaccine. The two companies have partnered to develop a therapy against the SARS-CoV-2 virus responsible for COVID-19. AbCellera also recently received more than $175 million from the Canadian government to discover solutions for COVID-19 and to build a manufacturing facility for antibody therapies against future pandemic threats.
In silico clinical trials could be especially useful today in the search for a vaccine for COVID-19. Drug discovery and development, from initial proof-of-concept to commercial launch—is a years-long complex process entailing enormous capital costs. With the savings in labor, costs, and time that can be achieved with in silico testing, researchers may be able to identify and develop drug candidates more quickly in the battle against COVID-19, where time is of the essence.
In silico explained
In the continuing quest to develop new drugs that are safe and effective, clinical research sees two methodologies in prevalent use today. Both represent alternatives to the traditional in vivo clinical trial model involving live test subjects.
The first approach is human organs-on-chips—microfluidic devices lined with living human cells that mimic the workings of a human organ and possible disease states to predict the efficacy and toxicity of drugs in development—which Vamstar will cover in more detail in a future Insight article. The second is in silico, the focus of this Insight piece, which also tackles the related question of harnessing AI for drug discovery.
The term in silico clinical trials indicates the use of individualized computer simulation in the development or regulatory evaluation of a medical product or intervention, including pharmaceuticals. The simulations may involve CT or MRI scans of real patients, which are converted into 3D anatomical shapes. Statistical modeling then enhances the data by creating anatomy scenarios.
Also known as computational medicine, in silico modeling, simulation, and visualization can help predict the chances for success of medicinal compounds and drugs being tested while also bringing to light possible adverse side effects during the drug discovery process.
This is the rationale for conducting clinical trials—to determine whether a product is safe and effective. Often, however, no explanations are provided, nor are any recommendations given to improve outcomes if results from the clinical trials prove unsuccessful. Products with potential or promise end up being abandoned, even when a modification may be all that is needed.
Such a severe, all-or-nothing approach discourages the search for answers and dooms innovation. As a result, fewer new biomedical products make it to the market, thereby increasing development costs overall, in turn raising the risk exposure for investors.
The costs from an abandoned clinical trial are sobering. A clinical trial may well be in an advanced stage of testing when harmful side effects or other issues are discovered or come to light. In 9 out of 10 instances, candidate therapies typically fail somewhere between the Phase I trial period and the final step in the regulatory approval process.
Consider the following. Pharmaceutical companies allocate an average of 17% of their total annual revenue to research and development. In 2019, pharmaceutical makers with a high R&D percentage of their revenue allocation included Sweden’s AstraZeneca, at 26%; US maker Eli Lilly, at 22%; and French-based Roche, at 21%. Next, development of the new drug could entail an additional $1 billion to $3 billion, including costs related to abandoned trials.
This is also why it is increasingly difficult for companies to undertake the development of new products that target rare conditions—either because the associated costs cannot be justified given the limited return on investment; or because the resulting sale price of the new drug would be so high as to pose a challenge for universal healthcare systems.
Advantages and benefits of in silico
Under the current system for drug discovery and testing, incremental changes can be made to existing technology, or altogether new technologies can be integrated. While completely simulated clinical trials are not feasible at present given the current technology, in silico clinical trials are expected to have significant benefits over current in vivo clinical trials, where testing is performed on living organisms, such as animals or humans.
The main advantage of in silico trials lies in its simulated or virtual context: the drugs being tested are carried out in a virtual setting involving virtual patients, involving no live humans or animals. With computer modeling and simulation, the possible consequences—therapeutic benefits as well as adverse side effects—of a drug regimen can be observed and even predicted. Thus, in silico trials could protect public health by saving consumers from debilitating side effects or undesirable drug interactions. In silico can also help advance more personalized medicine by letting doctors try out treatment plans. And unlike traditional testing involving live subjects, virtual human models can be reused indefinitely, delivering significant cost savings.
Already, the US Food and Drug Administration (FDA) advocates the use of in silico modeling and simulation because it can help pave the way for the development of safe and effective new therapeutics.
A decade’s worth of developments
Several developments can be noted relating to in silico clinical trials over the last decade, beginning with the FDA’s release in 2011 of a strategic plan governing computer modeling and simulation, up until South Korea’s announcement in December 2019 of a national AI strategy involving drugs and their targets. These developments are summarized in the infographic below.
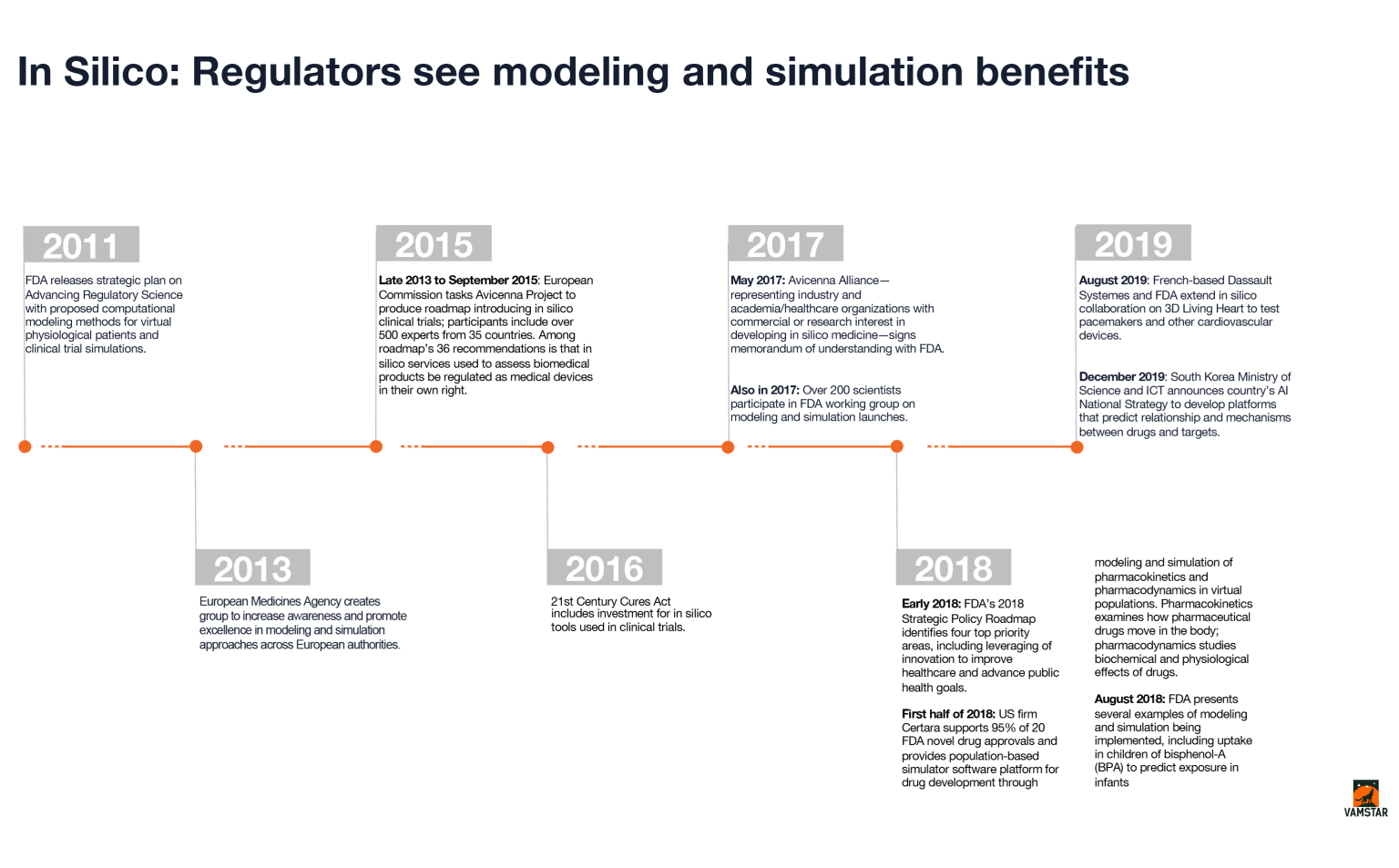
AI and in silico
Frequently used as interchangeable terms, AI and in silico also possess important distinctions. AI attempts to construct computer systems that emulate human problem-solving behavior through algorithms that learn. In this sense, AI can be utilized in various ways to improve drug discovery.
In silico modeling and simulation is a multidisciplinary field that includes systems and software engineering, computer science, but not necessarily AI. As to clinical trials, a goal of in silico technologies is the reduction of product trials on animals and humans. In silico models are also used in pharmacology for digital twins in bioprocesses.
The use of AI to develop new drugs is a field that has been rapidly developing. The analyst team at Los Angeles-based market research firm Village, which recently updated its list of vendors offering AI tools and services for drug discovery as well clinical diagnostics, has identified more than 260 such vendors that are active. And many have recently partnered with big pharma, including Atomwise and Strateos in California, Berg and GNS Healthcare in Massachusetts, Insilico Medicine in Maryland, Recursion Pharmaceuticals in Utah, England’s DeepMatter and Exscientia, and South Korea’s Standigm, to name a few.
In the case of Recursion Pharmaceuticals, the company entered into a global licensing agreement with Japan’s Takeda Pharmaceutical to gain rights to a clinical-stage MEK inhibitor—the chemical or drug with the potential to treat some cancers—and to develop it for the treatment of a hereditary cancer syndrome.
AI is applied to drug discovery at different points in the development cycle, a round that includes data mining, preclinical development, lead discovery, target identification, clinical development, drug safety, and drug repurposing. As to the actual drug production, AI plays a part in digital biomanufacturing, in which data management, data modeling, automation, and AI tools are used for process optimization. Digital twins of bioprocesses have started to gain traction in the biopharmaceutical industry as well, in which laboratory experiments are replaced with in silico simulations, enabling a relatively inexpensive and fast environment for research and development.
In silico players: Group 1
Players in the in-silico space can be categorized into two. In the first group are companies and organizations that are directly involved in simulation development, including outfits such as Certara, Dassault Systemes, GNS Healthcare, Novadiscovery, Nuventra Pharma Sciences, and Siemens. This group also includes organizations such as the In Silico Oncology Group, the Virtual Physiological Human Institute, and the Osteoporotic Virtual Physiological Human Project. Details of each follow below.
US firm Certara’s research into the in-silico evaluation of bioequivalence and bioavailability has been actively encouraged by the FDA. In June 2019, the company announced that its Simcyp physiologically based pharmacokinetic (PBPK) modeling and simulation technology was used to demonstrate bioequivalence for FDA approval of a complex generic drug on the agency’s abbreviated new drug application (ANDA) pathway. In February 2020, Certara acquired a range of modeling and simulation technology assets from Massachusetts-based In Silico Biosciences.
French-based Dassault Systemes had extended as of August 2019 its collaboration with the FDA for a project on virtual patients based on computational modeling and simulation to improve the efficiency of clinical trials for new device designs. The project involves a simulated 3D heart model.
Massachusetts firm GNS Healthcare’s causal AI technology integrates a wide variety of patient data types into in silico patients, revealing the complex system of interactions underlying disease progression and drug response. Virtual patients enable the simulation of drug response at the individual patient level.
French entity Novadiscovery provides an in silico clinical trial simulation platform that blends disease models and real-world data from both clinical trials and preclinical work, enabling relatively small amounts of data to be used when predicting clinical trial outcomes.
Nuventra Pharma Sciences of North Carolina provides pharmacokinetic simulations, among other in silico services.
Siemens of Germany is evaluating in silico clinical trials for medical devices with virtual patients, in which CT or MRI scans of real patients are converted into 3D anatomical shapes.
HumMod provides a top-down model of human physiology—from whole organs to individual molecules, including variables such as body fluids, circulation, electrolytes, hormones, metabolism, and skin temperature. Various research projects already use HumMod to create a heterogeneous population with thousands of virtual patients by randomly varying physiological parameters. HumMod is exclusively licensed to HC Simulation, LLC, by the University of Mississippi Medical Center.
The In Silico Oncology Group in Athens, Greece, is developing an in silico experimental platform, as well as an advanced medical decision support tool called Oncosimulator, in collaboration with several research centers in Europe and Japan to optimize cancer treatment. The oncosimulator is an integrated software system simulating in vivo tumor response to therapeutics within a clinical trial environment.
The Virtual Physiological Human Institute, incorporated in Belgium, is an international non-profit organization with cross-industry collaboration, whose mission is to ensure that the Virtual Physiological Human is fully realized, universally adopted, and effectively used in both research and clinical settings. Various medical fields can collaborate through the VPH framework. For example, the Cardioproof initiative brought together more than 170 cardiologists to create new, personalized tools for the management of congenital heart disease through advanced computer models.
The Osteoporotic Virtual Physiological Human Project, a European initiative, is working to predict the risk of fractures in patients with low bone mass.
As noted in the Avicenna Project report, the biomedical industry already uses simulation in the drug development process. Even so, most use cases are in the early stage of the development cycle, and thus have limited impact on cost, as the largest portion of expenses is tied to the clinical trials.
To be sure, one goal of in silico clinical trials is to reduce animal and human experimentation throughout the different drug trial phases. But before that can happen, more confidence must be established with healthcare providers and regulators—an ongoing process.
Part of the solution is to undertake more research projects that compare results obtained in silico with those obtained in vivo. Positive results should eventually wear away at any cultural resistance to in silico, like the healthcare industry’s initial tentative uptake of cloud services and AI for diagnostics, with uptake in both areas now starting to accelerate.
In silico players: Group 2
In the second group of in silico players are those involved in screening and manufacturing processes, including names such as Abzena, BioNTech, Codexis, Creative BioLabs, Go Silico, and Insilico Biotechnology. Details of each follow below.
Abzena, from England, is a contract development and manufacturing organization using in silico to identify potential sequence liabilities that could lead to late-stage manufacturing problems. The company also has in silico tools to screen antibodies and proteins for potential immunogenicity.
BioNTech Small Molecules GmbH, in Germany and part of BioNTech SE, provides lead discovery and “hit identification” through in silico screening. Hit identification is the first step for a drug discovery project where the identified ‘hit’, or valid small molecule, binds to the target and modifies its function.
Codexis Inc., in California, and the company’s CodeEvolver product, provides in silico, high-throughput assay screening (with AI) to enable DNA sequencing of new biotherapeutics.
Creative BioLabs, based in New York, provides an in silico method for target screening. The technique offers the potential not only to identify compound-target interactions and biochemical mechanisms, but also to investigate drug repurposing.
Go Silico, from Germany, provides ChromX, a simulation software that enables development of preparative liquid chromatographic downstream processes. The software relies on the simulation of mechanistic models, where a system is understood by the workings of its distinct parts and the way they are integrated.
Insilico Biotechnology, also from Germany, is developing digital twins to enable product quality through virtual experimentation.
Pharmaceuticals | AI | In Silico | Computational Medicine | Regulatory Science | In Silico Biotechnology | Artificial Intelligence | Machine Learning | Simulation | BioNTech | Abzena | Codexis Inc | ChromX | DNA Sequencing | Osteoporotic Virtual Physiological Human Project | GNS Healthcare | Drug Discovery

